We found 48 results that contain "post jpg"
Posted on: #iteachmsu


ADD post
HISTORY OF ROOT CAUSE ANALYSIS
Root cause analysis can be traced to the broader field of total quality management (TQM). TQM has developed in different directions, including a number of problem analysis, problem solving, and root cause analysis.
Root cause analysis can be traced to the broader field of total quality management (TQM). TQM has developed in different directions, including a number of problem analysis, problem solving, and root cause analysis.
Posted on: #iteachmsu


Avoid squandering a post-COVID-19 fish bounty
Unlike other investments, living ocean resources literally grow during downturns. During World War II, many fishing vessels were forced to stop fishing. This reprieve allowed fish populations, such as cod, to increase. Should any such gains be accruing during COVID-19, we must resist the urge to immediately over-harvest them. Instead, we should use fisheries science to design intelligent harvest-yield protocols that maximize the long-term benefit of any possible COVID-19 gains.
NAVIGATING CONTEXT
Posted on: #iteachmsu


Greek POST
If you're trying to learn Greek Articles you will find some useful resources including a course about Definite and Indefinite Articles... to help you with your Greek grammar. Try to concentrate on the lesson and notice the pattern that occurs each time the word changes its place. Also don't forget to check the rest of our other lessons listed on Learn Greek. Enjoy the rest of the lesson!
Authored by: chathu
Posted on: #iteachmsu

Full blood count
Department of Haematology
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Authored by: Vijaya
Posted on: #iteachmsu


Full blood count 1
Department of Haematology
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Posted by: Super Admin
Disciplinary Content
Posted on: #iteachmsu

Department of Haematology
Department of Haematology
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Posted by: Super Admin
Assessing Learning
Posted on: #iteachmsu


Full blood counts -- New
Department of Haematology
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Authored by: Vijaya
Disciplinary Content
Posted on: #iteachmsu

Department of Haematology
Department of Haematology
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Notes
Full blood counts are performed on automated equipment and provide haemoglobin concentration, red cell indices, white cell count (with a differential count) and platelet count.
The presence of abnormal white cell and red cell morphology is flagged by the analysers.
Blood films may be inspected to confirm and interpret abnormalities identified by the cell counter, or to look for certain specific haematological abnormalities.
Grossly abnormal FBC results and abnormal blood films will be phoned through to the requestor.
There is no need to request a blood film to obtain a differential white count. It is, however, important that clinical details are provided to allow the laboratory to decide whether a blood film, in addition to the automated analysis, is required.
Under some circumstances a differential is not routinely performed, e.g. pre-op, post-op, antenatal and postnatal requests.
Full Blood Counts are performed at CGH and GRH
See also: Reticulocyte Count
The FBC comprises the following tests
Standard
Haemoglobin (Hb)
White Blood Count (WBC)
Platelet Count (Plt)
Red Cell Count (RBC)
Haematocrit (HCT)
Mean Cell Volume - Red cell (MCV)
Mean Cell Haemoglobin (MCH)
Differential White Cell Count (where applicable)
Neutrophils
Lymphocytes
Monocytes
Eosinophils
Basophils
And if appropriate
Blood Film
Sample Requirements
2ml or 4ml EDTA sample or a Paediatric 1ml EDTA sample.
Sample Storage and Retention
Pre analysis storage: do not store, send to laboratory within 4 hours.
Sample retention by lab: EDTA samples are retained for a minimum of 48 hours at 2-10°C
Transport of samples may affect sample viability, i.e. FBC results will degenerate if exposed to high temperatures, such as prolonged transportation in a hot car in summer.
This test can be added on to a previous request as long as there is sufficient sample remaining and the sample is less than 24 hours old.
Turnaround Times
Clinical emergency: 30 mins
Other urgent sample: 60 mins
Routine: within 2 hours
Reference Ranges
If references ranges are required for paediatric patients please contact the laboratory for these.
Parameter Patient Reference Range Units Haemoglobin Adult Male 130 - 180 g/L Adult Female 115 - 165 g/L Red Cell Count Adult Male 4.50 - 6.50 x10^12/L Adult Female 3.80 - 5.80 x10^12/L Haematocrit Adult Male 0.40 - 0.54 L/L Adult Female 0.37 - 0.47 L/L Mean Cell Volume Adult 80 - 100 fL Mean Cell Haemoglobin Adult 27 - 32 pg White Cell Count Adult 3.6 - 11.0 x10^9/L Neutrophils Adult 1.8 - 7.5 x10^9/L Lymphocytes Adult 1.0 - 4.0 x10^9/L Monocytes Adult 0.2 - 0.8 x10^9/L Eosinophils Adult 0.1 - 0.4 x10^9/L Basophils Adult 0.02 - 0.10 x10^9/L Platelet Count Adult 140 - 400 x10^9/L
Posted by: Super Admin
Posted on: #iteachmsu
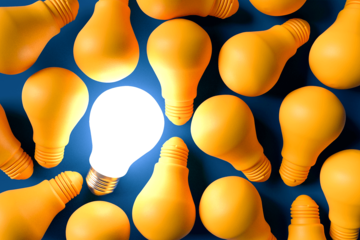
In our last post, We had a close look at Credentialing and what it entails. We also gained insight into how healthcare companies and providers manage this very important function in healthcare recruitment. Having understood why healthcare credential management is so crucial not only from a business perspective but also ensures there are no legal implications, the stage is just right to introduce another factor closely related to Credentialing, namely Compliance.
Join me in exploring why Compliance in Credentialing is so important and how this need not be such an onerous task with specialized apps, customized specifically for online healthcare recruitments. Credential compliance is achievable with minimal stress. Let us understand how, but first-a brief background.
What is Compliance in Credentialing, and Why does it matter?
I am using the the term ‘Compliance’ to mean meeting the requirements for Credentialing and participating in effective Compliance programs as set forth by the Office of Inspector General (OIG) and the National Committee for Quality Assurance(NCQA). This includes internal auditing, monitoring, credentialing education and training, developing plans of corrective action in responding to related problems as well as enforcing credentialing standards. Most Compliance programs, while generally operating as independent entities, report to their respective boards of directors or other committees providing assistance and oversight to the process.
So, what happens if a healthcare fails to verify accurately? Without careful oversight and auditing, it is all too possible for omissions or errors to occur before, during, or immediately following the process, which could lead to enrollment issues as well as open a pandora’s box to legal problems if the process is incomplete or the provider’s privacy is compromised. Furthermore, the 1960s case of Darling vs. Charleston Hospital established the responsibility of hospitals and other healthcare facilities in verifying the professional credentials of the physicians and other providers practicing under their roof.
REF : links :https://targetrecruit.com/the-importance-of-compliance-in-credentialing/
YouTube: https://youtu.be/C6YrPt1ygX8

THE IMPORTANCE OF COMPLIANCE IN CREDENTIALING
In our last post, We had a close look at Credentialing and what it entails. We also gained insight into how healthcare companies and providers manage this very important function in healthcare recruitment. Having understood why healthcare credential management is so crucial not only from a business perspective but also ensures there are no legal implications, the stage is just right to introduce another factor closely related to Credentialing, namely Compliance.
Join me in exploring why Compliance in Credentialing is so important and how this need not be such an onerous task with specialized apps, customized specifically for online healthcare recruitments. Credential compliance is achievable with minimal stress. Let us understand how, but first-a brief background.
What is Compliance in Credentialing, and Why does it matter?
I am using the the term ‘Compliance’ to mean meeting the requirements for Credentialing and participating in effective Compliance programs as set forth by the Office of Inspector General (OIG) and the National Committee for Quality Assurance(NCQA). This includes internal auditing, monitoring, credentialing education and training, developing plans of corrective action in responding to related problems as well as enforcing credentialing standards. Most Compliance programs, while generally operating as independent entities, report to their respective boards of directors or other committees providing assistance and oversight to the process.
So, what happens if a healthcare fails to verify accurately? Without careful oversight and auditing, it is all too possible for omissions or errors to occur before, during, or immediately following the process, which could lead to enrollment issues as well as open a pandora’s box to legal problems if the process is incomplete or the provider’s privacy is compromised. Furthermore, the 1960s case of Darling vs. Charleston Hospital established the responsibility of hospitals and other healthcare facilities in verifying the professional credentials of the physicians and other providers practicing under their roof.
REF : links :https://targetrecruit.com/the-importance-of-compliance-in-credentialing/
YouTube: https://youtu.be/C6YrPt1ygX8
Authored by: Greg
Disciplinary Content
Posted on: #iteachmsu

ABOUT
Teaching Commons: “an emergent conceptual space for exchange and communityamong faculty, students, and all others committed to learning as an essential activity of life in contemporary democratic society”(Huber and Hutchings, 2005, p.1)
What Is the #iteachmsu Commons? You teach MSU. We, the Academic Advancement Network, The Graduate School, and The Hub for Innovation in Learning and Technology, believe that a wide educator community (faculty, TAs, ULAs, instructional designers, academic advisors, et al.) makes learning happen across MSU. But, on such a large campus, it can be difficult to fully recognize and leverage this community’s teaching and learning innovations. To address this challenge, the #iteachmsu Commons provides an educator-driven space for sharing teaching resources, connecting across educator networks, and growing teaching practice.#iteachmsu Commons content may be discipline-specific or transdisciplinary, but will always be anchored in teaching competency areas. You will find blog posts, curated playlists, educator learning module pathways, and a campus-wide teaching and learning events calendar. We cultivate this commons across spaces. And through your engagement, we will continue to nurture a culture of teaching and learning across MSU and beyond.
How Do I Contribute to the #iteachmsu Commons? Content is organized by posts, playlists and pathways.Posts: Posts are shorter or longer-form blog postings about teaching practice(s), questions for the educator community, and/or upcoming teaching and learning events. With an MSU email address and free account signup, educators can immediately contribute blog posts and connected media (e.g. handouts, slide decks, class activity prompts, promotional materials). All educators at MSU are welcome to use and contribute to #iteachmsu. And there are no traditional editorial calendars. Suggested models of posts can be found here.Playlists: Playlists are groupings of posts curated by individual educators and the #iteachmsu community. Playlists allow individual educators to tailor their development and community experiences based on teaching competency area, interest, and/or discipline.Pathways: Pathways are groupings of educator learning modules curated by academic and support units for badges and other credentialing.
There are two ways to add your contribution to the space:Contribute existing local resources for posts and pathways: Your unit, college, and/or department might already have educator development resources that could be of use to the wider MSU teaching and learning community. These could be existing blog posts on teaching practice, teaching webinars, and/or open educational resources (e.g classroom assessments, activities). This content will make up part of the posts, playlists, and pathways on this site. Educators can then curate these posts into playlists based on their individual interests. Please make sure to have permission to share this content on a central MSU web space.Contribute new content for posts: A strength of the #iteachmsu Commons is that it immediately allows educators to share teaching resources, questions and events through posts to the entire community. Posts can take a variety of forms and are organized by teaching competency area categories, content tags, date, and popularity. Posts can be submitted by both individual educators and central units for immediate posting but must adhere to #iteachmsu Commons community guidelines.Posts could be:About your teaching practice(s): You discuss and/or reflect on the practices you’re using in your teaching. In addition to talking about your ideas, successes, and challenges, we hope you also provide the teaching materials you used (sharing the assignment, slidedeck, rubric, etc.)Responses to teaching ideas across the web or social media: You share your thoughts about teaching ideas they engage with from other media across the web (e.g. blog posts, social media posts, etc.).Cross-posts from other teaching-related blogs that might be useful for the #iteachmsu community: You cross-post content from other teaching-related blogs they feel might be useful to the #iteachmsu community.About teaching-related events: You share upcoming teaching related events as well as their thoughts about ideas they engage with events at MSU and beyond (e.g. workshops, conferences, etc.). If these events help you think in new ways about your practice, share them with the #iteachmsu community.Questions for our community: You pose questions via posts to the larger community to get ideas for their practice and connect with others considering similar questions.What Are the #iteachmsu Commons Policies?Part of the mission of the #iteachmsu Commons is to provide space for sharing, reflecting, and learning for all educators on our campus wherever they are in their teaching development. The commons is designed to encourage these types of interactions and reflect policies outlined by the MSU Faculty Senate. We maintain the right to remove any post that violates guidelines as outlined here and by MSU. To maintain a useful and safer commons, we ask that you:Follow the MSU Guidelines for Social Media.Engage across the #iteachmsu commons in a civil and respectful manner. Content may be moderated in accordance with the MSU Guidelines for Social Media.Do not share private or confidential information via shared content on the #iteachmsu Commons.Content posted on the #iteachmsu Commons is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International license. Learn more about this licensing here. Posted comments, images, etc. on the #iteachmsu Commons do not necessarily represent the views of Michigan State University or the #iteachmsu Commons Team. Links to external, non-#iteachmsu Commons content do not constitute official endorsement by, or necessarily represent the views of, the #iteachmsu Commons or Michigan State University.What if I Have #iteachmsu Commons Questions and/or Feedback?If you have any concerns about #iteachmsu Commons content, please email us at iteach@msu.edu. We welcome all feedback and thank you for your help in promoting a safer, vibrant and respectful community.
What Is the #iteachmsu Commons? You teach MSU. We, the Academic Advancement Network, The Graduate School, and The Hub for Innovation in Learning and Technology, believe that a wide educator community (faculty, TAs, ULAs, instructional designers, academic advisors, et al.) makes learning happen across MSU. But, on such a large campus, it can be difficult to fully recognize and leverage this community’s teaching and learning innovations. To address this challenge, the #iteachmsu Commons provides an educator-driven space for sharing teaching resources, connecting across educator networks, and growing teaching practice.#iteachmsu Commons content may be discipline-specific or transdisciplinary, but will always be anchored in teaching competency areas. You will find blog posts, curated playlists, educator learning module pathways, and a campus-wide teaching and learning events calendar. We cultivate this commons across spaces. And through your engagement, we will continue to nurture a culture of teaching and learning across MSU and beyond.
How Do I Contribute to the #iteachmsu Commons? Content is organized by posts, playlists and pathways.Posts: Posts are shorter or longer-form blog postings about teaching practice(s), questions for the educator community, and/or upcoming teaching and learning events. With an MSU email address and free account signup, educators can immediately contribute blog posts and connected media (e.g. handouts, slide decks, class activity prompts, promotional materials). All educators at MSU are welcome to use and contribute to #iteachmsu. And there are no traditional editorial calendars. Suggested models of posts can be found here.Playlists: Playlists are groupings of posts curated by individual educators and the #iteachmsu community. Playlists allow individual educators to tailor their development and community experiences based on teaching competency area, interest, and/or discipline.Pathways: Pathways are groupings of educator learning modules curated by academic and support units for badges and other credentialing.
There are two ways to add your contribution to the space:Contribute existing local resources for posts and pathways: Your unit, college, and/or department might already have educator development resources that could be of use to the wider MSU teaching and learning community. These could be existing blog posts on teaching practice, teaching webinars, and/or open educational resources (e.g classroom assessments, activities). This content will make up part of the posts, playlists, and pathways on this site. Educators can then curate these posts into playlists based on their individual interests. Please make sure to have permission to share this content on a central MSU web space.Contribute new content for posts: A strength of the #iteachmsu Commons is that it immediately allows educators to share teaching resources, questions and events through posts to the entire community. Posts can take a variety of forms and are organized by teaching competency area categories, content tags, date, and popularity. Posts can be submitted by both individual educators and central units for immediate posting but must adhere to #iteachmsu Commons community guidelines.Posts could be:About your teaching practice(s): You discuss and/or reflect on the practices you’re using in your teaching. In addition to talking about your ideas, successes, and challenges, we hope you also provide the teaching materials you used (sharing the assignment, slidedeck, rubric, etc.)Responses to teaching ideas across the web or social media: You share your thoughts about teaching ideas they engage with from other media across the web (e.g. blog posts, social media posts, etc.).Cross-posts from other teaching-related blogs that might be useful for the #iteachmsu community: You cross-post content from other teaching-related blogs they feel might be useful to the #iteachmsu community.About teaching-related events: You share upcoming teaching related events as well as their thoughts about ideas they engage with events at MSU and beyond (e.g. workshops, conferences, etc.). If these events help you think in new ways about your practice, share them with the #iteachmsu community.Questions for our community: You pose questions via posts to the larger community to get ideas for their practice and connect with others considering similar questions.What Are the #iteachmsu Commons Policies?Part of the mission of the #iteachmsu Commons is to provide space for sharing, reflecting, and learning for all educators on our campus wherever they are in their teaching development. The commons is designed to encourage these types of interactions and reflect policies outlined by the MSU Faculty Senate. We maintain the right to remove any post that violates guidelines as outlined here and by MSU. To maintain a useful and safer commons, we ask that you:Follow the MSU Guidelines for Social Media.Engage across the #iteachmsu commons in a civil and respectful manner. Content may be moderated in accordance with the MSU Guidelines for Social Media.Do not share private or confidential information via shared content on the #iteachmsu Commons.Content posted on the #iteachmsu Commons is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International license. Learn more about this licensing here. Posted comments, images, etc. on the #iteachmsu Commons do not necessarily represent the views of Michigan State University or the #iteachmsu Commons Team. Links to external, non-#iteachmsu Commons content do not constitute official endorsement by, or necessarily represent the views of, the #iteachmsu Commons or Michigan State University.What if I Have #iteachmsu Commons Questions and/or Feedback?If you have any concerns about #iteachmsu Commons content, please email us at iteach@msu.edu. We welcome all feedback and thank you for your help in promoting a safer, vibrant and respectful community.
Posted by: Chathuri Super admin..
Assessing Learning
Posted on: #iteachmsu


Consequence:
Post creation for hyperlink issue : row 102
Post creation for hyperlink issue : row 102
Posted by: Chathuri Super admin..
Disciplinary Content
Posted on: #iteachmsu

Second post
: Artificial intelligence (AI) refers to the simulation of human intelligence in machines that are programmed to think like humans and mimic their actions. The term may also be applied to any machine that exhibits traits associated with a human mind such as learning and problem-solving.
: Artificial intelligence (AI) refers to the simulation of human intelligence in machines that are programmed to think like humans and mimic their actions. The term may also be applied to any machine that exhibits traits associated with a human mind such as learning and problem-solving.
Posted by: Roni Smith
Navigating Context
Posted on: #iteachmsu

Post 2 https://www.lipsum.com/ Why do we use it? It is a long established fact that a reader will be distracted by the readable content of a page when looking at its layout. The point of using Lorem Ipsum is that it has a more-or-less normal distribution of letters, as opposed to using 'Content here, content here', making it look like readable English. Many desktop publishing packages and web page editors now use Lorem Ipsum as their default model text, and a search for 'lorem ipsum' will uncover many web sites still in their infancy. Various versions have evolved over the years, sometimes by accident, sometimes on purpose (injected humour and the like).
Posted by: Greg Thomsan
Posted on: #iteachmsu

This micro-credential is aligned to the following Educators Rising Standards:
I. Understanding the Profession
II. Learning About Students
VII. Engaging in Reflective Practice
I. Understanding the Profession
II. Learning About Students
VII. Engaging in Reflective Practice
Posted by: Greg Thomsan
Assessing Learning
Posted on: #iteachmsu


What is social distancing?
Social distancing, also called “physical distancing,” means keeping a safe space between yourself and other people who are not from your household.To practice social or physical distancing, stay at least 6 feet (about 2 arms’ length) from other people who are not from your household in both indoor and outdoor spaces.
Social distancing should be practiced in combination with other everyday preventive actions to reduce the spread of COVID-19, including wearing masks, avoiding touching your face with unwashed hands, and frequently washing your hands with soap and water :
Social distancing, also called “physical distancing,” means keeping a safe space between yourself and other people who are not from your household.To practice social or physical distancing, stay at least 6 feet (about 2 arms’ length) from other people who are not from your household in both indoor and outdoor spaces.
Social distancing should be practiced in combination with other everyday preventive actions to reduce the spread of COVID-19, including wearing masks, avoiding touching your face with unwashed hands, and frequently washing your hands with soap and water :
Posted by: Chathuri Super admin..
Disciplinary Content
Posted on: #iteachmsu


https://www.google.com/search?rlz=1C5GCEA_enLK882LK883&biw=1350&bih=700&ei=HczNX5C3BYfgrQGuvCc&q=nature&oq=nature&gs_lcp=CgZwc3ktYWIQA1Dk4bkBWKfquQFg2Ou5AWgBcAB4AIABAIgBAJIBAJgBAKABAaoBB2d3cy13aXqwAQDAAQE&sclient=psy-ab&ved=0ahUKEwjQuZu-n7vtAhUHcCsKHS7eCQAQ4dUDCA0&uact=5
Posted by: Greg Thomsan
Assessing Learning
